Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2026-01-31 09:09:34
- wham09
- Member
- Registered: 2025-09-19
- Posts: 51
Solvent participation after transition state
Dear Prof. Lu,
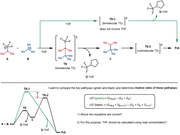
I'd like to ask for your advice about how to calculate the free energy change of a solution-phase reaction when solvent coordination seems to occur after the transition state.
For convenience of understanding what I mean, I attached the reaction diagram, explanations, and questions as a figure.
My questions are all in the figure, but I will also paste them below.
4. What would be the correct way here?
4-1) THF coordination to D happens after this reaction. dG = (G_C + G_D) – (G_A + G_B)
4-2) THF coordination to D happens during product formation. dG = (G_C + G_D-THF) – (G_A + G_B + G_THF)
5. If 4-2 is correct, should G_THF be calculated using the concentration as 12.3 M (neat solvent molarity) rather than 1.0 M? (when plugging into the dG equation)
Thank you very much in advance.
Last edited by wham09 (2026-01-31 22:31:56)
Offline
#2 2026-02-01 23:39:34
Offline
#3 2026-02-02 02:04:03
- wham09
- Member
- Registered: 2025-09-19
- Posts: 51
Re: Solvent participation after transition state
Thank you for the answer.
To explain my purpose more specifically, I attached another figure here, with a subsequent chemical step and an additional pathway.
Basically, my confusion is coming from the fact that the reactants and the final product are exactly same in the two pathways, but the different "timing" of the THF complexation (to BF3) in the one-step pathway and the two-step pathway.
Could you please further clarify the additional two questions that are included in the figure?
Offline
#4 2026-02-03 23:49:00
Re: Solvent participation after transition state
I seems that the elemenary step of generating D-THF is missing in the energy profile map. There should be a weakly interacting complex like TS'...THF, which corresponds to a PES minimum, and the TS' is the (unstable) molecule produced after passing through TS. THF will react with TS' to yield D-THF. Only when the complete reaction profile is constructed, the reaction rate can be estimated.
Offline