Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2025-10-08 08:30:51
- mishgan
- Member
- From: Germany
- Registered: 2025-10-08
- Posts: 2
ETS-NOCV analysis. Polar bond cleavage
Hello everyone!
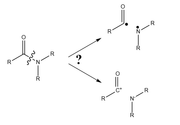
I needed to analyze the interaction of a substituent with the carbonyl oxygen in an amide. The manual only gives an example with ethane (ETS-NOCV analysis), where the bond is broken homolytically. Am I correct in assuming that the CN bond must be broken heterolytically for a proper analysis?
Last edited by mishgan (2025-10-08 08:31:37)
Offline
#2 2025-10-08 09:32:43
Re: ETS-NOCV analysis. Polar bond cleavage
ETS-NOCV can be used to analyze both the two bond-breaking manners, the choice of the status of the fragments are fully dependent of your purpose. If you hope to characterize the bonding nature in the molecule, usually the status of the two fragments should be chosen as close as possible to their actual status in the molecule; as C-N is not a ionic bond, homolytic form is more suitable for study.
Offline
#3 2025-10-08 10:17:07
- mishgan
- Member
- From: Germany
- Registered: 2025-10-08
- Posts: 2
Re: ETS-NOCV analysis. Polar bond cleavage
I agree that the bond is not ionic, but it is clearly more polarized than in the example in the manual. Moreover, the degree of polarization can vary depending on the substituent. Are there any recommendations at what point it would be more correct to describe a system as a product of heterolytic bond cleavage, rather than homolytic?
Thank you very much for your answer!
Offline
#4 2025-10-09 06:41:44
Offline