Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2024-07-06 21:08:05
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Very strange AIM results!
Dear Tian,
Please consider the below interacting systems:
A) CH2=NH(+)-O(-)....BCl3 B) CH2=NH(+)-S(-)....BCl3 C) CH2=NH(+)-Se(-)....BCl3
in which the hetero atom O, S, and Se (from dipole) interact with boron atom of BCl3. Binding energies are (kcal/mol)
-28.30(A)>-19.56(B)>-16.60(C). The QTAIM descriptors at the X...B BCP (X=O, S, Se) are:
Rho/au.u DelSquareRho/a.u. V G |V|/G H
A 0.1419 0.5532 -0.3472 0.2427 1.430 -0.1045
B 0.1190 -0.1836 -0.1549 0.0545 2.82 -0.1004
C 0.1089 -0.1813 -0.0957 0.0252 3.80 -0.0705
As can be seen, only Rho and H are in line with the trend found for strength interaction based on the value of interaction energies. In this case the Sign of DelSquarRho, and |V|/G are not in line with our expectation about NCI, a Pure CI, a pure electrostatic and so on. For instance, for C with smallest value of Rho, |V|/G is significantly exceeds 2 characterizing a pure covalent X...B interaction!
Could you please let me know your highly professional comments about these strange and confusing data?
Sincerely,
Saeed
Offline
#2 2024-07-07 10:16:26
Re: Very strange AIM results!
Dear Saeed,
Se is most polarizable among the O, S and Se, and Laplacian of rho at BCP is negative, implying that Se...B has evident covalent character, you can use Multiwfn to plot ELF or LOL to better verify this point, and compare contribution of orbital interaction by sobEDA analysis. It is not fully surprising that |V|/G at BCP significantly exceeds 2.
Best,
Tian
Offline
#3 2024-07-07 17:33:51
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: Very strange AIM results!
Dear Tian,
Many many thanks for your highly valuable and informative comments.
Please, if possible, let me clarify my mean more evident.
Based on values of binding energy, the O...B bonding provides the most strength interaction, S...B and Se...B are in the second and third place. This energetics trend is well satisfied by the value of Rho and H. Indeed, the values of Rho are quite consistent with the binding energy. Moreover, H is more negative for B...O than for S...B and Se...B. All these descriptors reveal that O...B bonding is more covalent than S...B which, in turn, is more covalent than Se...B. But, the challenging descriptor is V/G. We know that as the covalent character increases, V/G shifts toward greater values. For a non-covalent interaction. a partly covalent interaction, and a pure covalent interaction V/G is less than 1, between 1 and 2 and, greater than 2, respectively.
Thus, considering the trend of covalent character, B...O>B...S>B...Se, V/G shows a quite opposite trend! Please let me know why?
Meantime, I did perform IQA analysis. This analysis confirms that the B...X (X=O,S,Se) interaction, E_int(X,B), becomes more covalent as O<S<Se (V_XC increases and V_cl decreases). Indeed, from the covalent character point of view, the Se...B is the most covalent interaction and S...B and O...B are located in second and third place: Se...B>S...B>O...B. The IQA results are quite opposite to the QTAIM descriptors. Please let me know your valuable and professional explanations about such inconsistency.
In advance, too many thanks for your kind attention and please excuse me for bothering you, my nice friend.
Sincerely,
Saeed
Offline
#4 2024-07-08 00:44:02
Re: Very strange AIM results!
Dear Saeed,
I strongly suggest plotting plane map of H, Laplacian of rho, ELF using Multiwfn to visually examine the covalent character, also sobEDAw is useful. BCP is just one point, whose information sometimes lead to misleading conclusion.
Best,
Tian
Offline
#5 2024-07-08 18:20:55
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: Very strange AIM results!
Dear Tian,
Many many thanks for, as always, your highest kind attention to guide me with much valuable, professional, and novel comments. Please excuse me for a delayed reply; I was out of city during the past day and I had no access to the forum.
Please, be aware that I did perform sobEDA and the results confirm the covalent nature (characterized with delta_E_orb) of these interactions as O...B>S...B>Se...B which is in quite disagreement with IQA analysis. Meantime, ELF analysis proofs the covalent character as, again, O...B>S...B>Se...B. These various inconsistencies made me quite confused!
Sincerely,
Saeed
Offline
#6 2024-07-09 01:52:43
Re: Very strange AIM results!
Dear Saeed,
It is better to upload ELF plane map as well as Laplacian of rho plane map for the case of O...B, S...B, Se...B, so that I can clearly understand their characters. Also, it is best to explicitly provide E_orb values for the cases.
I do not comment IQA result.
Best,
Tian
Offline
#7 2024-07-09 17:41:34
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: Very strange AIM results!
Dear Tian,
While your highly kind attention, very valuable time, and energy are deeply and extremely appreciated, the data you have ordered is very respectfully presented in the attached "AA.pdf" document. Moreover, please be aware that a compressed folder is respectfully presented including the M06-2X-D3(0)-SMD(Dichloromethane)/6-311++G(d,p) wavefunction of the fully optimized structure of complexes (formed between three different 1,3-dipoles involving O, S, Se hetero atoms and BCl3 as LA catalysts) at the same level for your any further considerations.
Sincerely,
Saeed
Last edited by saeed_E (2024-07-09 19:35:39)
Offline
#8 2024-07-10 05:54:46
Re: Very strange AIM results!
Dear Saeed,
I can confirm that O-B is much more covalent than O-Se. If you calculate fuzzy bond order for O-B, you will find the bond order is close to 0.9, clearly indicating its strong covalency. In contrast, the bond order of O-Se is not so large. As you mentioned, this is also supported by sobEDA analysis.
Regarding why some BCP properties such as Laplacian of rho give contradictory conclusion, you can understand it easily by plotting contour line map of Laplacian of rho, see below image for the case of O-B. It can be seen that there is an evident electron density concentration area, as highlighted by red circle, clearly indicating its prominent covalent character. However, since the BCP occurs in the region that far away from the actual bonding region, the Laplacian of rho at the BCP is positive. It obviously doesn't mean that the O-B interaction doesn't show evident covalent character.
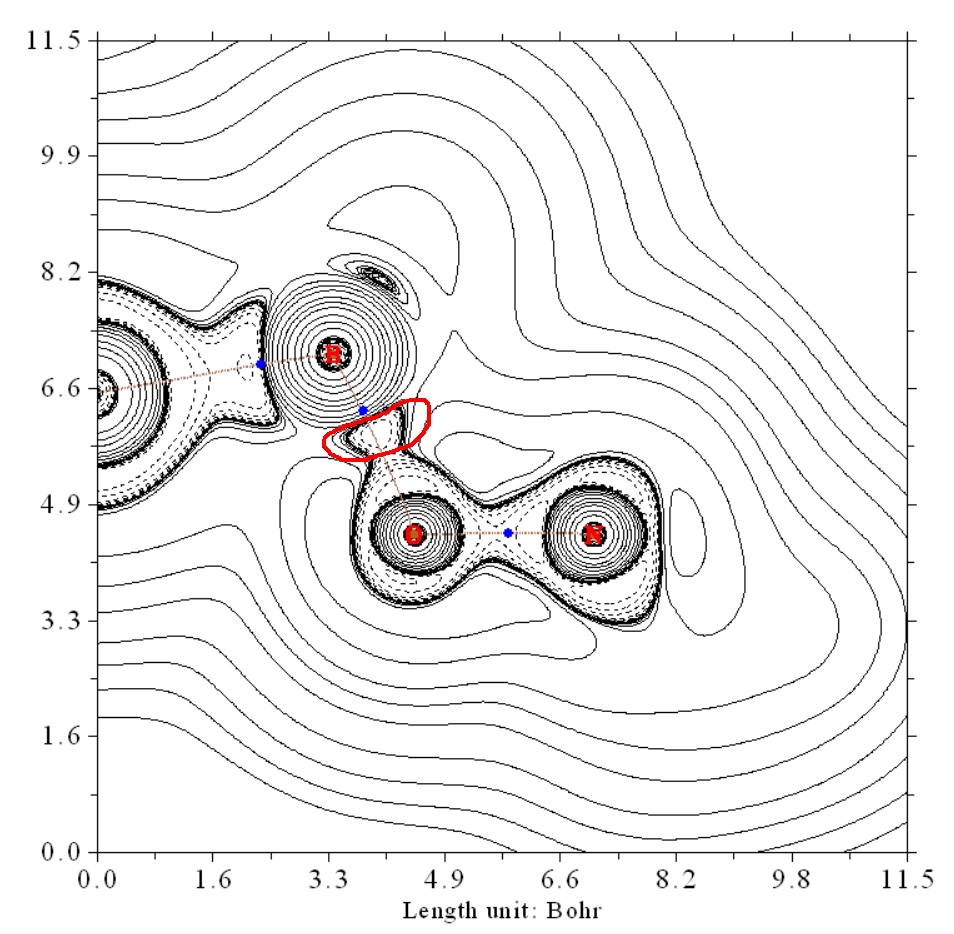
Best,
Tian
Offline
#9 2024-07-10 07:10:23
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: Very strange AIM results!
My nice and full of knowledge friend, dear Tian,
Many many thanks for your highest kind attention to prompt reply to my message and make me guide with highly professional and novel comments. Really, I was extremely enjoyed, surprised, and learned by your rationalizations. Undoubtedly, except you, nobody can explain this situation in such professional and exact manner.
Since these calculations are part of my current study for which too many calculations have been performed and a great part of time has been spent to write corresponding manuscript, I still need more of your guidance and please kindly let me respectfully send you more explanations and questions (through your email address) regarding this nice and interesting project whose results could be attractive for readers.
I deeply hope you can find free time to have a brief look at this email and, again, make me beneficial from the sea of your novel knowledge.
I will prepare this email soon and will send to you.
Sincerely yours,
Saeed
Offline