Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2024-06-08 02:10:06
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
ETA-NOCV on a cycloadduct-Singlet fragments or triplet fragments?
Dear Tian,
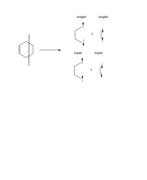
Please suppose one wants to study orbital interactions (bond strength) in cyclohexene. To this end, two different bonds breaking can be considered. Two single bonds should be broken. These breakings have shown in the attached picture. If possible, please have a look and so kindly let me know which approach is reasonable and why? Evidently, one approach should overestimate energetically C-C bonds formation in cyclohexene. Why?
As can be seen, the total number of alpha and beta spins in cyclohexen is equal to that of fragments and there is not any problems.
I am interesting in such a question since it seems activation/strain analysis can directly be applied over two competitive cycloadducts to fine why one is more stable than other one (taking separate reactants, one can find strain is main player or interaction).
Last edited by saeed_E (2024-06-08 02:23:01)
Offline
#2 2024-06-09 14:41:16
Re: ETA-NOCV on a cycloadduct-Singlet fragments or triplet fragments?
Dear Saeed,
This first scheme is slightly more reasonable, as in this case each fragment is in actual ground state electronic structure.
Best,
Tian
Offline
#3 2024-06-09 18:22:30
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: ETA-NOCV on a cycloadduct-Singlet fragments or triplet fragments?
Dear Tian,
Many many thanks for your highly valuable and informative comments, my nice friend.
Interestingly, I, myself, was thinking the same as your comment which both fragments should be in singlet state.
Indeed, if you also agree and confirm its reasonableness, the type of fragmentation is quite dependent on what goal we are following. Indeed, if we take separate fully optimized reagents as reference (which both are in singlet state), following the reaction, corresponding TS and cycloadduct are also generated at singlet state. Consequently, in the cycloadduct that two fragments are strongly bound, we should take separated fragments at singlet. Undoubtedly, ETS-NOCV analysis over the cycloadduct (product) should also be performed using two singlet fragments. I would be highly grateful if you kindly give me your valuable opinion on my statement, my nice friend.
Sincerely,
Saeed
Offline