Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2024-01-24 18:11:31
- brunosamp
- Member
- Registered: 2023-04-21
- Posts: 22
Help with NCI analysis with integration
I performed a nci analysis for a system of graphene oxide with a ammonia molecule interacting with the edge and basal hydroxyl.
In the NCI analysis, i observed that the interaction with the edge hydroxyl is described by a hydrogen bond region and weak van der walls interactions with the carbons near the hydroxyl. The same goes for the basal hydroxyl.
When i perform the integration for this structure, i got a list of domains. In the edge, one of the domains showed only the hydrogen bond interaction, and not the van der walls interaction. On the basal hydroxyl, i got a domain with the van der walls interaction and the hydrogen bond.
How can i get the exact same isosurface i got in the NCI analysis, when i do the integration?
Just to add context: the bond strength with the edge hydroxyl is higher than with the basal hydroxyl. But, with these domains i am getting, the integral of the interaction with the basal hydroxyl is higher than with the edge hydroxyl, which i understand it should be the opposite.
I used the default criterion for defining the domain (<0.5).
Last edited by brunosamp (2024-01-24 18:18:27)
Offline
#2 2024-01-24 18:15:48
Re: Help with NCI analysis with integration
one of the domains showed only the hydrogen bond interaction, and not the van der walls interaction
I don't know how did you obtain this conclusion, please clarify this point
Also, it is best to show me the NCI isosurface map so that I can better understand your situation.
Offline
#3 2024-01-24 18:34:22
- brunosamp
- Member
- Registered: 2023-04-21
- Posts: 22
Re: Help with NCI analysis with integration
Dear tian lu, i made a mistake while typing.
For the basal hydroxyl, i got a hydrogen bond interaction and a van der walls interaction as well.
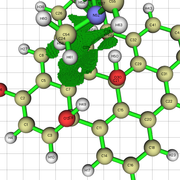
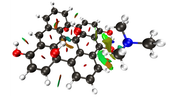
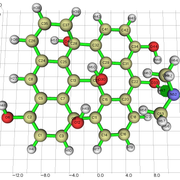
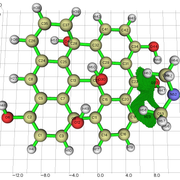
NCI Isosurface: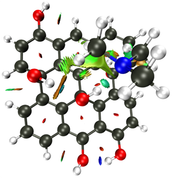
Closest domain to the NCI Isosurface i got, which described very well the NCI isosurface.
While for the edge hydroxyl, i got this nci isosurface (VdW interaction and the hydrogen bond with the edge hydroxyl):
And this domain was the closest one i got:
But in the nci, the hydrogen bond and vdw interaction are not separeted, like above. So, since the interaction with the edge hydroxyl is the strongest, i understand the integral value will not be well comparable with the integral value for the basal hydroxyl i showed above, i believe.
Offline
#4 2024-01-25 17:56:55
Re: Help with NCI analysis with integration
Both H-bond interaction and vdW interaction are evident for the two structures. If you only want to compare strength of H-bond, I would suggest using Multiwfn to perform AIM topology analysis to estimate H-bond interaction energy based on electron density at bond critical point of the H-bond using the relationship proposed in J. Comput. Chem. 2019, 40, 2868–2881, see Section 4.2.1 of Multiwfn manual for example.
If you want to study overall interaction strength, I would suggest directly calculating binding energy using quantum chemistry codes.
Offline
#5 2024-01-27 15:47:47
- brunosamp
- Member
- Registered: 2023-04-21
- Posts: 22
Re: Help with NCI analysis with integration
I Will do this sir, thank you very much
Offline