Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2021-02-25 16:27:59
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
dissociation asymptote
Dear all,
I'm calculating the bond dissociation energy of a complex system, hence, homolysis bond dissociation can not be used.
I used the following method
ex: if my molecule in CH4 change on of the C-H bond and calculate energy
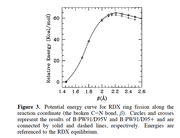
The plot of Energy vs C-H distance gives BDE at one point.
My question is whether we can find the details of dissociation asymptote to make sure that the correct open-shell fragments have obtained using multiwfn.
Thanks and regards.
Offline
#2 2021-02-26 03:50:19
Re: dissociation asymptote
I don't fully understand your meaning. To calculate BDE of C-H bond of CH4, you should calculate enthalpy for CH4, CH3 and H respectively, then BDE = H(CH3) + H(H) - H(CH4), this study is not directly relevant to Multiwfn, and you do not need to calculate and plot "Energy vs C-H distance".
In addition, I don't know why "homolysis bond dissociation cannot be used".
Offline
#3 2021-03-03 07:48:42
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: dissociation asymptote
Actually, I'm using this method to calculate bond dissociation energy since my complexes are salts. This salt optimization in the gas phase is very difficult. So I have incorporated the solvent effect.
My question is after dissociation can I check whether it has formed open-shell fragments using multiwfn
Offline
#4 2021-03-03 10:09:41
Re: dissociation asymptote
You can plot spin density or calculate atomic spin population using Multiwfn. If the fragment is closed-shell, they will be zero on the fragment atoms.
Note that to calculate BDE, you do not need to perform IRC task as the figure at all, and it is never a correct way of calculating BDE. You simply need to calculate enthalpy of the whole system and that of each fragment and obtain their difference.
Offline
#5 2021-03-04 00:46:58
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: dissociation asymptote
Thank you very much for your comment !!!
Offline
#6 2021-03-30 01:53:47
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: dissociation asymptote
Dear Tian,
When using enthalpy shall I use enthalpy of formation or just the enthalpy (Sum of electronic and thermal Enthalpies)
Offline
#7 2021-03-30 02:57:33
Re: dissociation asymptote
Dear Tian,
When using enthalpy shall I use enthalpy of formation or just the enthalpy (Sum of electronic and thermal Enthalpies)
The fully depends on your research purpose, they are quite different.
For calculating BDE purpose, you should use enthalpy
Offline
#8 2021-03-30 09:03:02
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: dissociation asymptote
Yes I need to calculate BDE
So I will use Sum of electronic and thermal Enthalpies of radicals and molecule and get the difference
Thanks a lot
Offline
#9 2021-03-31 05:15:59
- shenaya
- Member
- Registered: 2019-10-16
- Posts: 34
Re: dissociation asymptote
Yes I need to calculate BDE
So I will use Sum of electronic and thermal Enthalpies of radicals and molecule and get the difference
Thanks a lot
Offline