Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2021-02-26 23:36:57
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
A question about two competitive Halogen-bond interactions...
Dear Tian,
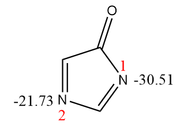
Please consider the attached image. This compound has two nitrogen atoms 1 and 2 which can participate in the intermolecular halogen-bond (XB) interaction toward a Lewis acid such as F-Cl (the Cl atom is the XB-donor). As can be seen, the value of V_s,min has been calculated for both basic centers N1 and N2. Since the value of V_s,min for N1, -30.51 kcal/mol, is significantly more negative than that of N2, -21.73 kcal/mol, one can expect that XB interaction between F-Cl and N1 should be stronger than that between F-Cl and N2. Considering some XB strength descriptors such as value of counterpoise-corrected interaction energy (CP-IE), value of rho, |V|/G, and H (V+G) at corresponding BCPs, all indicate that N2 whose V_s,min is less negative than that of N1 forms a stronger XB with Cl atom of F-Cl.
It seems, in this case, values of unperturbed V_s,min (values of V_s,min in the isolated Lewis base prior to participate in XB interaction) are not appropriate for a reasonable and true prediction of the strength of two XB interactions. In other words, in this case, polarization of negative sites by the electric field of positive sigma-hole of Cl (leading to the perturbed values of V_s,min) should be taken into account. To this end we can employ positive point charge approach.
Please, if possible, kindly let me know whether my rationalization is reasonable about the trend observed for the strength of these two XBs or there is any mistake.
In advance, your kind attention is very appreciated.
Sincerely,
Saeed
Offline
#2 2021-02-27 08:03:30
Re: A question about two competitive Halogen-bond interactions...
Dear Saeed,
Correct, difference in polarizability of the two nitrogens should be taken into account, the so called positive point charge method may be useful in explaning the difference. Also it is worth to consider plotting electron density map, and the HELP and HELV indices illustrated in Section 4.17.8 of Multiwfn manual may also be useful in understanding difference of the lone pairs of the two nitrogens.
Best regards,
Tian
Offline
#3 2021-02-27 08:58:50
- saeed_E
- Member
- Registered: 2019-12-21
- Posts: 334
Re: A question about two competitive Halogen-bond interactions...
Dear Tian,
Too many thanks for your kind attention, my nice friend.
As always, you so kindly gift me very valuable and informative comments as well as a novel guidance. I am happy that my interpretation is found correct. I will perform point charge analysis together with HELP and HELV. If you kindly let me, I will contact with you if any problem/question is encountered particularly regarding HELP and HELV indices which are going to be considered for the first time.
Yours sincerely,
Saeed
Offline