Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2023-07-12 03:13:44
- Nanocubes
- Member
- Registered: 2023-07-12
- Posts: 2
Question on the calculation of hydrogen bond strength
Hi everyone,
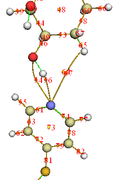
I am trying to calculate the strength of hydrogen bond between a pyridine derivative and an alcohol using topology analysis (4.2.1.) for the optimized geometry using Gaussian. According to 4.2.1, the hydrogen bond strength is calculated from the electron density at BCP. However, the electron density at BCP between H (of OH group) and N (of pyridine) is only around 0.00534, which results in the bond strength being only ~1.89 kJ/mol. I feel this value is too low for a hydrogen bond, may I know if there's any other way to perform such calculation?
I also notice that there is a BCP connecting O (of OH group) and N (of pyridine). I'm not sure if this can be counted towards the hydrogen bond? (because it's very close to the BCP between H and N). If it can be counted, does it mean that my hydrogen bond strength will be equal to the sum of binding energy calculated for individual BCP?
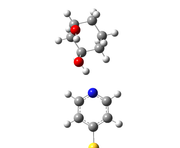
I also attached the image of optimized geometry and BCPs.
Thank you!
Offline
#3 2023-07-12 09:37:54
- Nanocubes
- Member
- Registered: 2023-07-12
- Posts: 2
Re: Question on the calculation of hydrogen bond strength
Hi Prof, thanks for your reply. Indeed I use CP56 to evaluate H-bond strength, but the density of all electrons is only 0.00534 (so the energy calculated using this method is only ~1.9 kJ/mol).
If I use the Potential energy density V(r)= -0.02265 (mentioned in the manual (4.2.1) but not recommended), then the binding energy will be ~29 kJ/mol, which sounds more reasonable.
For now I will try to read J. Comput. Chem., 40, 2868 (2019) and Chem. Phys. Lett., 285, 170 (1998) to understand more about this. Please let me know if any other references are recommended.
Thank you again for your time!
Offline