Multiwfn forum
Multiwfn official website: http://sobereva.com/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
Pages: 1
#1 Re: Multiwfn and wavefunction analysis » Help for using ELF in Coordination Compounds. » 2023-04-30 16:50:01
Dear Tian Lu.
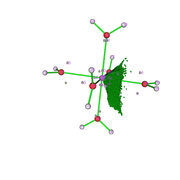
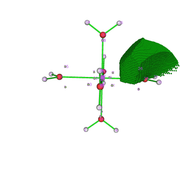
I fully understood the effect of the grid value on the analysis. Thanks. I reevaluated the data and it made more sense. I still have two doubts: 1) V(Zn,O) in the figure that describes the attractor 19 (fig1) and the attractors 26 and 9 would the V(O) represent the isolated pairs (fig 2 is atractor 26 which is close to atractor 9)? The electron density analysis matches this assessment.
2) Is there a DI value for the BPC obtained by QTAIM? Should this be calculated using the BPC coordinate by redoing the basin analysis in the option Study source function in AIM basins?
#2 Multiwfn and wavefunction analysis » Help for using ELF in Coordination Compounds. » 2023-04-29 13:40:37
- glauciobf
- Replies: 3
I am evaluating the use of Multiwfn for comparative work on QTAIM and ELF, as found in recent papers on coordination compounds. So, I did a test and based on the models from the Multiwfn manuals. However, I have a doubt, when identifying the attractors in the ELF analysis that are generated from the electronic density. I do not identify points on the bond axis, as in the case of the QTAIM analysis. In fact, what I identify and what I have the impression is that they are the isolated pairs of the O atom of the water molecule and the orbitals of the metallic ion (Zn2+). Is this right? Because when trying to analyze this way, the result indicates a very low interaction at these points. Could anyone give any tips?
Do I need to make any corrections for proper analysis of attractors in the M-O bond?
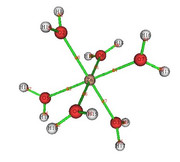
The aquacomplex [Zn(OH2)6]2+ was optimized with Functional M06L2X and base function Def2tzvp.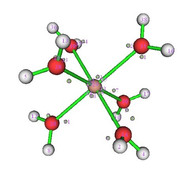
Pages: 1